Results 191 to 200 of 541
LinkBacks (?)
-
Untitled document
Refback This thread03-29-2014, 08:21 PM -
Untitled document
Refback This thread03-28-2014, 11:03 AM -
03-26-2014, 03:41 PM
-
Untitled document
Refback This thread03-18-2014, 03:00 PM -
Untitled document
Refback This thread03-18-2014, 12:22 PM -
Multiple independent tests confirmed that levels nuclear radiation detected Calif beaches more than 10x normal level : Cecalli_Helper
Refback This thread02-01-2014, 12:39 PM -
WHY IS'NT MSM REPORTING ON THE escalating DANGERS of Radiation from Fukushima - WASALive
Refback This thread01-05-2014, 02:45 PM -
01-05-2014, 10:26 AM
-
ALIPAC: WHY IS'NT MSM REPORTING ON THE escalating DANGERS of Radiation from Fukushima
Refback This thread01-04-2014, 05:46 PM -
01-04-2014, 04:21 PM
-
01-04-2014, 04:13 PM
Thread Information
Users Browsing this Thread
There are currently 1 users browsing this thread. (0 members and 1 guests)
-
02-17-2014, 08:40 AM #191AprilGuestI agree there might be nothing they can do to stop it, but they need to be tracking and reporting it so people can make an effort protect themselves from the effects of the radiation ...too lie and cover and distract is inexcusable IMO. This is a life or death situation and the crisis goes on......while the band plays on...we are in the twilight zone!I believe that the media stopped reporting on this because it is indeed a major catostrophic event. I think they don't want people to freak out. Truth is....I don't think there is anything they can do about it now that it has happened. We were warned by many that this could happen and it has. The reality is that we are going to have to live out (or die out) the consequences of this event.
-
02-17-2014, 08:42 AM #192
-
02-17-2014, 08:45 AM #193AprilGuest
36 Signs The Media Is Lying To You About How Radiation From Fukushima Is Affecting The West Coast
The west coast of the United States is being absolutely fried by radiation from the Fukushima nuclear disaster, and the mainstream media is not telling us the truth about this. What you are about to see is a collection of evidence that is quite startling. Taken collectively, this body of evidence shows that nuclear radiation from Fukushima is affecting sea life in the Pacific Ocean and animal life along the west coast of North America in some extraordinary ways. But the mainstream media continues to insist that we don’t have a thing to worry about. The mainstream media continues to insist that radiation levels in the Pacific and along the west coast are perfectly safe. Are they lying to us? Evaluate the evidence compiled below and come to your own conclusions…
#1 Independent researchers have measured alarmingly high levels of radiation on the beaches of the west coast. For example, the video posted below was taken on December 23rd, 2013 at Pacifica State Beach. As you can see in this video, radiation levels near the water are up to five times higher than normal background radiation…
#2 According to Oceanus Magazine, the total amount of cesium-137 that has been released into the Pacific Ocean from Fukushima is 10,000 to 100,000 times greater than the amount released into the oceans by the Chernobyl disaster or by the atmospheric nuclear weapons tests of the 1960s.
#3 Former MSNBC host Cenk Uygur has admitted that while he was at MSNBC he was instructed not to warn the public about the radiation coming from Fukushima…“I was on MSNBC at the time when this happened, I said, “Don’t trust what the Japanese government is saying, they’ll say trust what the electric power company is saying. Go, go, go, get outta there. Get as far away from that plant as you can. It’s literally a core meltdown.” And they always don’t want people to panic, so they were always like, “Oh it’s going to be okay.” [...] I’m like, “You’re crazy man, don’t be anywhere near that reactor.” And I remember at the time, of course not at The Young Turks, but on cable news, people were like, “Hey Cenk, you know, I don’t know that you want to say that, because the official government position is that it’s safe.” Oh, is that the official government position? Now go explain that to the people who served on the USS Ronald Reagan.”#4 71 U.S. sailors who assisted with the initial Fukushima relief efforts have developed serious diseases such as testicular cancer, thyroid cancer, Leukemia, “unremitting gynecological bleeding” and brain tumors since that time as a result of exposure to radiation coming from Fukushima.
#5 Something is causing starfish all along the west coast of the United States to literally disintegrate into piles of “white goo“…Researchers say nuclear pollution from the 2011 earthquake in Japan that damaged the Fukushima Nuclear Power Plant could be partially to blame for a disease wiping out starfish along the West Coast.#6 Bald eagles are dying in unprecedented numbers in Utah, and nobody can figure out why this is happening…
Dr. Peter Raimondi of the University of Santa Cruz says something is making starfish susceptible to whats believed to be a bacteria coined “Wasting Disease.” It essentially disintegrate the marine invertebrates into a white goo, after the starfish loses its legs.Bald eagles are dying in Utah — 20 in the past few weeks alone — and nobody can figure out why.#7 Huge numbers of dead birds are dropping dead and washing up along the coastlines of Alaska. It is being reported that many of the carcases of the dead birds are “broken open and bleeding”.
Hundreds of the majestic birds — many with wing spans of 7 feet or more — migrate here each winter, gathering along the Great Salt Lake and feasting on carp and other fish that swim in the nearby freshwater bays.
Earlier this month, however, hunters and farmers across five counties in northern and central Utah began finding the normally skittish raptors lying listless on the ground. Many suffered from seizures, head tremors and paralysis in the legs, feet and wings.
#8 The recent deaths of thousands of birds in Oregon is absolutely baffling scientists.
#9 Something is causing large numbers of seals and walruses up in Alaska to lose hair and develop “oozing sores”.
#10 Substantial numbers of polar bears along the coast of Alaska are suffering from fur loss and open sores.
#11 There is an epidemic of sea lion deaths along the California coastline.
#12 The population of sockeye salmon along the coastlines of Alaska is at a “historic low”.
#13 Something is causing Pacific herring to bleed from their gills, bellies and eyeballs.
#14 Dangerous levels of cesium-137 have been discovered in mushrooms and berries grown along the west coast.
#15 According to an absolutely shocking report put out by the National Academy of Sciences, it has been proven that Pacific Bluefin tuna have transported radioactive material “across the entire North Pacific Ocean”…“We report unequivocal evidence that Pacific Bluefin tuna, Thunnus orientalis, transported Fukushima-derived radionuclides across the entire North Pacific Ocean.”#16 Something seems to be causing a substantial spike in the death rate for killer whales living off of the coast of British Columbia.
#17 Experts have found very high levels of cesium-137 in plankton living in the waters of the Pacific Ocean between Hawaii and the west coast.
#18 One test in California found that 15 out of 15 Bluefin tuna were contaminated with radiation from Fukushima.
#19 Back in 2012, the Vancouver Sun reported that cesium-137 was being found in a very high percentage of the fish that Japan was selling to Canada…
• 73 percent of the mackerel
• 91 percent of the halibut
• 92 percent of the sardines
• 93 percent of the tuna and eel
• 94 percent of the cod and anchovies
• 100 percent of the carp, seaweed, shark and monkfish
#20 An EU-funded study concluded that Fukushima released up to 210 quadrillion becquerels of cesium-137 into the atmosphere.
#21 One very experienced Australian adventurer has stated that he felt as though “the ocean itself was dead” as he journeyed from Japan to San Francisco recently…The next leg of the long voyage was from Osaka to San Francisco and for most of that trip the desolation was tinged with nauseous horror and a degree of fear.#22 It is being projected that the radioactivity of coastal waters off the U.S. west coast could double over the next five to six years.
“After we left Japan, it felt as if the ocean itself was dead,” Macfadyen said.
“We hardly saw any living things. We saw one whale, sort of rolling helplessly on the surface with what looked like a big tumour on its head. It was pretty sickening.
“I’ve done a lot of miles on the ocean in my life and I’m used to seeing turtles, dolphins, sharks and big flurries of feeding birds. But this time, for 3000 nautical miles there was nothing alive to be seen.”
In place of the missing life was garbage in astounding volumes.
“Part of it was the aftermath of the tsunami that hit Japan a couple of years ago. The wave came in over the land, picked up an unbelievable load of stuff and carried it out to sea. And it’s still out there, everywhere you look.”
#23 The deputy chairman of Russia’s State Duma Committee for Natural Resources, Maxim Shingarkin, says that seafood captured off the northwest coast of the United States is so radioactive that it represents a “danger for mankind”…
#24 According to one recent scientific report, radiation from Fukushima could affect our seafood for “many generations” and ultimately kill more than a million people…This cycle will last for many generations, because of the food chain of fish and other marine fauna, and the radioactivity will be recycled and in fact the meat content will increase rather than decreasing by decay. Even if only one one-hundredth of the radioactivity (more than 1e15 Bq of CS137) were to enter this recirculation pattern, the collective whole body ingestion dose over many generations would exceed 1e7 Sv, sufficient to kill more than 1,000,000 people.#25 The Japanese government has estimated that approximately 300 tons of highly radioactive water is pouring into the Pacific Ocean from the destroyed Fukushima nuclear facility every single day.
#26 A senior researcher of marine chemistry at the Japan Meteorological Agency’s Meteorological Research Institute says that “30 billion becquerels of radioactive cesium and 30 billion becquerels of radioactive strontium” are being released into the Pacific Ocean from Fukushima every single day.
#27 According to Tepco, a total of somewhere between 20 trillion and 40 trillion becquerels of radioactive tritium have gotten into the Pacific Ocean since the Fukushima disaster first began.
#28 According to a professor at Tokyo University, 3 gigabecquerels of cesium-137 are flowing into the port at Fukushima Daiichi every single day.
#29 It is being projected that significant levels of cesium-137 will reach every corner of the Pacific Ocean by the year 2020.
#30 It has been estimated that the entire Pacific Ocean will soon “have cesium levels 5 to 10 times higher” than what we witnessed during the era of heavy atomic bomb testing in the Pacific many decades ago.
#31 The immense amount of radioactive material being released into the Pacific Ocean from Fukushima has caused environmental activist Joe Martino to issue the following warning…“Your days of eating Pacific Ocean fish are over.”#32 The Iodine-131, Cesium-137 and Strontium-90 that are constantly being released from Fukushima are going to affect the health of those living in the northern hemisphere for a very, very long time. Just consider what Harvey Wasserman had to say about this…Iodine-131, for example, can be ingested into the thyroid, where it emits beta particles (electrons) that damage tissue. A plague of damaged thyroids has already been reported among as many as 40 percent of the children in the Fukushima area. That percentage can only go higher. In developing youngsters, it can stunt both physical and mental growth. Among adults it causes a very wide range of ancillary ailments, including cancer.#33 Outdoor radiation levels at Fukushima recently hit a new all-time high.
Cesium-137 from Fukushima has been found in fish caught as far away as California. It spreads throughout the body, but tends to accumulate in the muscles.
Strontium-90’s half-life is around 29 years. It mimics calcium and goes to our bones.
#34 According to the Wall Street Journal, it is being projected that the cleanup of Fukushima could take up to 40 years to complete.
#35 Yale Professor Charles Perrow is warning that if the cleanup of Fukushima is not handled with 100% precision that humanity could be threatened “for thousands of years”…“Conditions in the unit 4 pool, 100 feet from the ground, are perilous, and if any two of the rods touch it could cause a nuclear reaction that would be uncontrollable. The radiation emitted from all these rods, if they are not continually cool and kept separate, would require the evacuation of surrounding areas including Tokyo. Because of the radiation at the site the 6,375 rods in the common storage pool could not be continuously cooled; they would fission and all of humanity will be threatened, for thousands of years.”#36 There are very alarming reports that new “unexplained plumes of radioactive steam” are rising at Fukushima. Japanese officials are not able to get inside and see what is causing these plumes. Some are speculating that the crisis at Fukushima just got a whole lot worse. The following is from a recent Ecologist article…Unexplained plumes of radioactive steam have been rising from Fukushima’s Reactor Building 3, Could a major meltdown be on the way?If a full-blown meltdown does happen at Fukushima, it would be an environmental disaster unlike anything that we have ever seen before in human history.
Fukushima’s Reactor Building 3 exploded on 13th March 2011 as a result of a hydrogen buildup, breaching the building’s containment and emitting a huge plume of radiation. The reactor itself is in meltdown.
And now fresh plumes of steam have been seen coming out the structure. These have now been confirmed by Tepco, the owner of the nuclear plant, from 19th December onwards. The company believes the steam is coming from the fifth floor of the building.
However it does not know the cause of the steam. Lethal levels of radiation and the physical damage to the structure have so far made entry and inspection impossible.
As we enter 2014, we are entering a time when the world is becoming increasingly unstable. The global economy is being shaken, political corruption is seemingly everywhere, evidence of advanced social decay is all around us and the earth itself is starting to groan and crack with increasingly regularity. But the mainstream media continues to insist that everything is going to be just fine. That is one of the reasons why I wrote my new novel. The American people deserve to hear the truth and be warned about the great challenges that are rapidly approaching.
In the end, millions upon millions of people could end up getting seriously ill as a result of all of this radiation coming from Fukushima. Most of them will never even know why they have gotten sick.
And if there is a major earthquake or a significant accident during the cleanup at Fukushima, we could actually see huge sections of Japan be evacuated permanently.
It would be hard to overstate just how serious all of this is. But you won’t hear about this from the mainstream media. Their story is that “everything is okay” and they are sticking to it.
Source: thetruthwins.com
-
02-18-2014, 08:38 AM #194AprilGuest
The US bans some of the agricultural products from Japan due to radiation contamination from Fukushima and does FDA undermine the potential dangers of low radiation?
Recently, South Korea’s top newspaper, The Dong-A-Ilbo reported that “Concerns over Japan’s radioactive contamination and its seafood is spreading to most countries in the Pacific basin”. South Korea has recently banned all the fishery imports from Japan and since 2011 other countries China have banned the import of vegetables, seafood and dairy products from at least 5 Japanese prefectures, including Fukushima.
Due to the health concerns of the public, FDA has increased surveillance of products from Japan and recently, United States has banned agricultural and fishery imports from 14 prefectures in Japan.
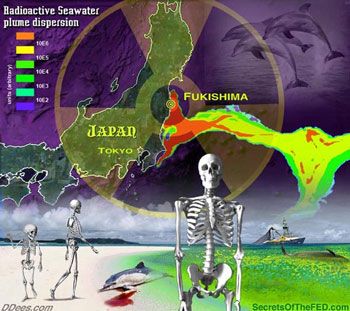
Although the Japanese government claim that the situation is ‘under control’, many other countries and researchers believe that Fukushima nuclear plant is at the state of emergency at the moment. Find out how Fukushima nuclear plant is now leaking between 150-250 tons of radioactive water into the Pacific Ocean daily.
According to FDA’s website, FDA is processing all food products from Japan in four categories:
Category 1: Products that the Japanese government has restricted for export or sale. (These products are prevented from entry to US):
- Prefectures, and dace, ayu, and cherry salmon (yamame) from Fukushima.
- Spinach, lettuce, celery, cress, endive, escarole, chard, collards, and other head-type leafy vegetables from the Fukushima Prefecture.
- Turnips and other non-head type leafy vegetables, as well as broccoli, cauliflower, flower head brassicas (i.e. broccoli and cauliflower), mushrooms bamboo shoots, and Ostrich fern from the Fukushima Prefecture.
- Sand lance from Fukushima Prefecture
- Milk from the Fukushima and Ibaraki Prefectures.
- Spinach and kakina from the Fukushima and Ibaraki Prefectures.
Category 2: Products from Fukushima, Ibaraki, and Tochigi Prefectures that Japanese government has not currently banned:
Under Import Alert 99-33, the authorities may detain dairy and fresh produce Fukushima, Ibaraki, and Tochigi Prefectures when they arrive in the U.S.
Category 3: Foods and feed products that are not covered by FDA’s Import Alert coming from these three Japanese Prefectures:
- Fukushima
- Ibaraki
- Tochigi
Category 4: All the other FDA-regulated food products from Japan that are not listed in the Import Alert and do not belong to other categories
Authorities will review these products using standard procedures, and as part of this may monitor and sample products as resources permit.
What about fish that swim from the reactor site into U.S. fishing waters?
According to FDA’s website, “Japan to U.S. waters would take several days under the best of circumstances. Vessels fishing in waters far off U.S. shores must also travel several days to return to port. It is unlikely that a fish exposed to significant levels of radionuclides near the reactor could travel to U.S. waters and be caught and harvested. If this improbable trip did occur, the level of short-lived radionuclides such as I-131 would drop significantly through natural radioactive decay during the time needed to make the journey. At this time, Japanese tests have detected longer-lived radionuclides such as Cs-137 in only a few samples and at levels below FDA DILs. FDA’s testing of fish imported from Japan has not detected the presence of Cs-137. In the unlikely scenario that pollutants could affect fish that have traveled to the U.S., FDA will work with the National Oceanic and Atmospheric Administration (NOAA) to test seafood caught in those areas. Together FDA and NOAA will also inspect facilities that process and sell seafood from those areas.”
However, according to the Swedish government who carefully monitored radioactivity from plant and animal foods, most animal products including meat, dairy and fish (especially cod, tuna and halibut that contain large amount of mercury) have higher radioactive substances compared to fruits, grains and vegetables. In fact, Tuna has the highest contamination since it travels around the entire Pacific Ocean, so it’s potentially more contaminated (with toxins, heavy metals and radiation) than wild salmon. On the other hand, wild salmon is very territorial and does not travel as much as tuna. It’s also safer to eat salmon from rivers of Canada like Copper River salmon or Coho salmon.

Is FDA undermining the potential dangers of low radiation by considering that radioactive fish is safe?
According to a report published in the International Journal of Health, large amounts of airborne radioactivity has been spread throughout Japan and other nations and there could have been more than 14,000 deaths in US related to Fukushima.
The report states that “Some samples of radioactivity in precipitation, air, water, and milk, taken by the U.S. government, showed levels hundreds of times above normal; however, the small number of samples prohibits any credible analysis of temporal trends and spatial comparisons. U.S. health officials report weekly deaths by age in 122 cities, about 25 to 35 percent of the national total. Deaths rose 4.46 percent from 2010 to 2011 in the 14 weeks after the arrival of Japanese fallout, compared with a 2.34 percent increase in the prior 14 weeks. The number of infant deaths after Fukushima rose 1.80 percent, compared with a previous 8.37 percent decrease. Projecting these figures for the entire United States yields 13,983 total deaths and 822 infant deaths in excess of the expected”.
The report also shows that although the radiation exposure in US have been far below those in Japan, the exposure to low radiation (that was previously assumed to be harmless) can cause solid tumors and leukemia, damage to the central nervous system and elevated disease rates in children born to women who underwent pelvic X-rays during pregnancy.
According to an article published in the Institute of Science in Society, Dr. Mae-Wan Ho, states that “low dose radiation is all the more dangerous because it does not kill the targeted cell, but allows its influence to spread widely to adjacent cells, thus multiplying the radiation effect (about 100 fold).”
Other studies including the one published in the National Academy of Sciences shows that even low exposure to radiation can increase the risk of many health problems and there is not threshold for radiation that can be considered safe or harmless.
Studies show that chronic fatigue syndrome, dizziness, back pain, early aging syndrome, muscle and joint pains, fever, cervical lymph node sensitivity and weight loss are some of other symptoms of exposure to low radiation.
http://www.seattleorganicrestaurants...-radiation.php
-
02-18-2014, 08:40 AM #195AprilGuest
Bystander Effects Multiply Dose & Harm from Ionizing Radiation
Effects of radiation felt by non-radiated neighbouring cells prompt a rethink of radiation risk, radiotherapy and radioprotection Dr. Mae-Wan Ho
A fully referenced version of this article is posted on ISIS members website and is otherwise available for download here
Please circulate widely and repost, but you must give the URL of the original and preserve all the links back to articles on our websiteLow dose big effects Special report to be included in Science in Society #55 (available August 2012). Pre-order now or Subscribe. All proceeds from SiS 55 will be donated to children of Fukushima and Chernobyl
Special report to be included in Science in Society #55 (available August 2012). Pre-order now or Subscribe. All proceeds from SiS 55 will be donated to children of Fukushima and Chernobyl
Linear dose response relationships are routinely used in risk assessments of exposure to environmental hazards, and ionizing radiation is no exception. Typically, effects at high doses that kill cells, cause gene mutations and cancers, are back extrapolated to obtain an exposure limit at which the harm caused is considered miniscule or acceptable in view of the benefits gained. Ionizing radiation was widely believed to cause mutations by directly breaking the bonds of DNA molecules in the nucleus.
In the early 1990s, Hatsumi Nagasawa and John Little at Harvard School of Public Health, Boston, Massachusetts, discovered, to their surprise, that while a linear relationship applies to high doses of a-radiation (from 5cGy to 1.2 Gy, where cGy = 10-2Gy) (see Box), a much enhanced effect was obtained at very low doses of 0.03 cGy to 0. 25 cGy, when 30 to 45 % of the cells in a population of Chinese hamster cells exhibited sister chromatid exchange (SCE involving double-stranded DNA breaks). At that low dose of radiation, only 0.07 to 0.6 % of the nuclei should have been directly hit by an alpha-particle. Yet the frequency of SCE rose rapidly at very low doses reaching a plateau below 1 cGy, after which no further increase occurred with increasing dose, though a decline occurred at higher doses. That was the first indication that damaging signals may be transmitted from irradiated to neighbouring non-irradiated cells in a population, and they called it “the bystander effect” [1].
In another experiment they looked at mutation frequency of a specific enzyme, and found the same enhanced effect at very low dose. At the lowest dose of 0.83 cGy, the efficiency with which the alpha-particle can induce a mutation increases nearly five-fold; the mutation frequency was the same as that due a dose 100 times as great (0.83 Gy).
Using the then newly developed microbeam of very low dose alpha particles to target individual cells, researchers at Columbia University, New York, showed that hitting the cytoplasm was sufficient to induce mutation in the nucleus [3]. They commented that low dose radiation is all the more dangerous because it does not kill the targeted cell, but allows its influence to spread widely to adjacent cells, thus multiplying the radiation effect (about 100 fold).
Bystander effects now abundantly confirmedAbsorbed dose, equivalent dose and effect dose
Radioactivity is measured physically as Curies (1 Ci = 3.7 x1010 disintegrations per second). But that does not take account of the energy of different kinds of radiation and their interaction with biological tissues.
The absorbed dose, Gray (Gy) is equal to and energy of 1 Joule/ kg absorbed.
The equivalent dose Sievert (Si), is weighted by biological potency of different kinds of radiation (1 for g-rays, b-particles, and X-rays, 20 for a-particles and 10 for neutrons). The effective dose also in Sievert takes into account the sensitivities of different tissues, applying weighting factors derived from previous epidemiological studies of radio-induced cancers. Thus, lots of judgements are used in arriving at the effective dose, based on a model of linear energy transfer (and linear dose response relationship) that has proven inapplicable for cells and organisms.
Since then, a wide range of bystander effects in cells not directly exposed to ionizing radiation have been found, which are the same as or similar to those in the cells that were exposed [4], including cell death and chromosomal instability.
Actually, radiation induced bystander effects have been described as far back as 1954, when factors that cause damage to chromosomes could be detected in the blood of irradiated patients. Carmel Mothersill and Colin Seymour at McMaster University published a key paper in 1997 showing that filtered medium from irradiated human epithelial cells can reduce the survival of unirradiated cells, suggesting that soluble factors produced by the irradiated cells were involved in the bystander effects [5].
Indeed, serum from cancer patients treated with radiotherapy also causes cell death and chromosomal instability in unexposed cells in culture, and this has been shown as far back as 1968 [6].
In 2001, researchers at Columbia University, New York used microbeams to target single cells with exactly defined numbers of a-particles. They found that hitting 10 % of the cells induced the same frequency of cancerous transformation as when every cell in the dish was targeted [7].
More recently, bystander DNA double-strand breaks were induced in a three-dimensional human tissue culture that is closer to in vivo conditions. The results obtained by the team led by Olga Sedelnikova at the National Cancer Institute, Bethesda, Maryland, were much more dramatic. In marked contrast to cultured cells in two-dimensions where maximal DSB occurred 30 minutes after irradiation, the incidence of DSBs in bystander cells reached a maximum between 12 to 48 hours after irradiation, gradually decreasing only over 7 days. At the maximum, 40 to 60 % of cells were affected [8]. These increases in bystander DSBs were followed by increased apoptosis and micronucleus formation, loss of nuclear DNA methylation and increased fractions of senescent cells. The authors commented that treatment of primary tumours with radiation therapy frequently results in the growth of a secondary malignancy of the same or different origin. They raised the question on whether bystander effects could introduce negative complications in radiation therapy, such as genomic instability in normal tissues. They concluded that induced senescence might be a protective mechanism. On the other hand, failure of these protective pathways can lead to the appearance of proliferating, damaged cells and to an increased probability of oncogenic transformation.
New research from the University of Pittsburgh Pennsylvania throws further light on the implications of bystander effects for radiotherapy. It is customary for patients receiving bone-marrow transplant to undergo whole body irradiation to kill the bone marrow cells of the host so as to encourage repopulation by transplanted cells. The researcher found that irradiated mouse recipients significantly impaired the long-term repopulating ability of transplanted mouse haematopoietic stem cells (HSCs) 17 hours after exposure to irradiated hosts, and before the cells began to divide. There was an increase in acute cell death associated with accelerated proliferation of the bystander HSCs. The effect was marked by a dramatic down-regulation of c-Kit (a proto-oncogene), apparently because of elevated reactive oxygen species (ROS). Administration of an antioxidant chemical or ectopically over-expression of a ROS scavenging enzyme catalase improved the function of transplanted HSCs in the irradiated hosts [9]. This obviously has implications for protecting patients during radiotherapy as well as those receiving bone-marrow transplant.
What causes the bystander effects?
The bystander effect is largely a low-dose phenomenon, appearing at doses below 10 cGy [10]. Higher doses often do not produce bystander effect possibly because the cells targeted are killed before they can influence non-targeted cells. As with the “war on cancer”, numerous attempts have been made to identify the genes or gene products involved in the bystander effects. And as in cancer, genes up-regulated or down-regulated are secondary to a state of electronic imbalance (see [11] Cancer a Redox Disease, SiS 54) created by the ionizing radiation, which breaks chemical bonds and generate free electrons (see Box 2).
When cells are irradiated, it is likely that ionization of one or more of the atoms on DNA molecules will occur in a direct hit, breaking the DNA chain or the links between chains. However, direct attack of radiation on the structure of DNA is not the only way radiation affect cells. The human body is about 70 % water; hence water is probably the most frequent target of ionizing radiation. Ionization of water leads to the formation of reactive oxygen species (ROS) (see Box 3) that damages DNA, lipids, proteins, carbohydrates, and other molecules. It is becoming increasingly clear that ROS is a major culprit in the bystander effect, as suggested by those who discovered the effect [1, 2]. This has been confirmed by more recent findings.Box 2
How ionizing radiation can impact on health
Ionizing radiation comes from radioactive decay of unstable chemical elements, which are generated in the nuclear fission process in nuclear power reactors, or in linear accelerators that produce X-rays and electron beams (b-particles) for radiotherapy [12, 13]. In general photons or particles with energy above 10 eV (electron volts) are ionizing.
Nuclear fission is the splitting of the nucleus of a large atom into two, along with a few neutrons and release of energy in the form of heat and g-rays; about 0.2 to 0.4 % of fissions also produce a-particles (nuclei of helium-4 with two protons and two neutrons), or nuclei of tritium (one proton and two neutrons). The fission products are often unstable and hence radioactive; they undergo b-decay giving out b-particles, antineutrinos, and additional g-rays. Antinutrinos pass easily through ordinary matter; consequently, the major ionising radiations that can affect health are a- and b-particles, X-rays, g-rays and neutrons.
a- and b-particles are directly ionizing radiation; they interact directly with atoms, and if the energy is sufficient, knock outer electrons away to produce a free electron and a positively charged ion. A b-particle produces more than 100 ionizing events per cm in its track, whereas an a-particle produces more than 10 000 ionizing events per cm. But while a b-particle can travel for centimetres through tissues, a-particles travel for micrometres only. As the energy of each particle increases, so does the range. Consequently, external sources of a-particles are stopped by the skin, while external b-particles can penetrate into the body. However, inhaled or ingested sources of a-particles can do a lot more damage within the body.
X-rays and g-rays induce ionization indirectly through 3 principal mechanisms: Compton scattering where they are scattered from the outer electrons of atoms, transferring energy to the electrons, and if enough energy is transferred, give rise to a free electron and a positively charged ion. In the photoelectric effect, one of the inner electrons of the atom absorbs the energy of the X-ray or g-ray, and is ejected from the atom, again leaving a positively charged ion. Following this, one of the outer electrons ‘falls’ in to fill the vacancy, and X-ray is emitted from the atom. In pair formation, the x-ray or g-ray interacts with the electric field of the nucleus, and is converted into an electron and a positron, the positron in travelling through the tissue material will usually react with another electron and become converted back to two X-rays or g-rays.
Neutrons are scattered directly from the atomic nuclei of atoms, resulting either in losing energy that is released as g-rays or else it is absorbed by the nuclei resulting in a new nucleus (element) being formed. If the new nucleus is unstable, radioactive decay occurs creating a-, b- or g-rays. The second option can only occur if the neutron is sufficiently slow, and that is what happens in the nuclear fission process in nuclear power reactors.
Some of the free electrons generated by the ionizing radiation may be energetic enough to cause ionizations of their own; this is the secondary photoelectron effect of ionizing radiation.
ROS and oxidized extracellular DNABox 3
Reactive oxygen species generated from water [14]
Oxygen is the most important electron acceptor in the biosphere. It readily accepts unpaired electrons to give rise to a series of partially reduced species collectively known as reactive oxygen species (ROS). These include superoxide O·2-, hydrogen peroxide H2O2, hydroxyl radical HO· and peroxyl radical OO·, which may be initiate and propagate free radical chain reactions damaging to cells. Hydroxyl radicals are generated by ionizing radiation either directly from water, or indirectly by the formation of secondary partial ROS that are subsequently converted to hydroxyl radicals by metabolic processes. Gamma rays, beta and alpha particles are all able to ionize water to produce hydroxyl radicals, the most reactive, and therefore potentially the most hazardous. Hydroxyl radicals have a very short persistence time, while hydrogen peroxide is the most long-lasting. Hydrogen peroxide can diffuse freely and can generate hydroxyl radicals by reacting with free electrons:
H2O2 + e- → HO· + HO- (1)
Oxidative attack on proteins destroys their enzyme, receptor and other biological function; damage to DNA causes mutations and chromosomal rearrangements; and peroxidation of lipids destroys membrane structure and function.
More than 80 % of energy of ionizing radiation deposited in cells results in the ejection of electrons from water. Subsequent reactions with surrounding water results in the formation of several reactive species: eaq- (hydrated free electron) HO· (hydroxyl radical, the most important reactive oxygen species), H· (hydrogen radical), H2 (hydrogen gas) and H2O2 (hydrogen peroxide, a stable and diffusible reactive oxygen species). These products react rapidly with each other and with surrounding molecules. In the presence of O2, superoxide radicals (another reactive oxygen species) are formed:
eaq- + O2 → O·2- (1)
H· + O2 → O·2- + H+ (2)
Superoxide generates hydrogen peroxide on a longer time scale:
2 O·2- + H+ → O2 + H2O2 (3)
Because of their instability, most of the reactions generating the primary radical products will have taken place within 1 millisecond, but superoxide and H2O2 will persist and diffuse to more distant sites.
Cellular damage by hydroxyl radical attack depends partly on the antioxidant status of the cell and partly on the availability of reducing systems capable of reducing or activating superoxide or hydrogen peroxide. The cellular antioxidant status determines the intracellular concentration of ROS. It has been shown that the effects of H2O2 resemble those of ionizing radiation. Cells exhibiting high levels of SOD, catalase, and peroxidase activity are relatively less vulnerable to secondary effects of radiation. Glutathione peroxidase catalyses the reaction:
H2O2 + 2 GSH (reduced glutathione) → 2 H2O + GSSG (oxidized glutathione) (4)
The activity of this peroxidase depends on the availability of reduced GSH. Regeneration of GSH from GSSG by glutathione reductase requires reduced nicotinamde adenine dinucleotide phosphate (NADPH) as electron donor.
The hydroxyl radical can be produced from more stable ROS via the participation of an electron donor, and many transition metal ions can act as electron donors:
H2O2 + Fe (II) → Fe(III) + HO- + HO· (5)
Thus, hydroxyl radicals are generated from H2O2 at sites where reduced transition metals are present.
A team led by Aleksei Ermakov at the Research Centre for Medical Genetics, Russian Academy of Medical Sciences in Moscow Researchers showed that an extracellular DNA (ecDNA) derived from the cell genome participates in the bystander effect induced by X-ray exposure in human lymphocytes and human umbilical-vein epithelial cells [15]. Their previous work suggested that radiation-sensitive cells undergoing apoptosis serve as a source of ecDNA fragments that diffuse in the medium and bind to DNA receptors on the surface of bystander cells. Bystander effects could be stimulated by ecDNA of irradiated cells but not by ecDNA produced by normal cells. In a new study, the team tested the idea that the difference between the two types of ecDNA is due to DNA oxidation events occurring during and after irradiation. They compared the production of NO (nitric oxide, a free radical and reactive oxygen species) and ROS in human endothelial cells that were irradiated at a low dose radiation, or exposed to the ecDNAR extracted from the media conditioned by irradiated cells, or exposed to the genomic DNA oxidized in vitro by treatment with H2O2, (DNAo1), or H2O2 plus uv light (DNAO2more strongly oxidizing). They found that all three treatments gave similar responses. The production of NO at 2h was suppressed at low doses of 0.03 Gy and 0.1 Gy but increased at 0.5 Gy or higher. Similarly, the ecDNAR extracted from media conditioned by irradiated cells decreased NO but not the extracellular DNA from non-irradiated cells; the oxidized DNA o1 and more so DNAO2 also reduced NO. ROS levels in general were increased in all three treatments by 1.2 to 1.8-times the controls with ecDNAR and oxidized DNA o1 and DNAO2 to larger extents than the direct radiation, or the bystander effect due from the conditioned medium.
Other researchers have shown that the major source of ROS in endothelial cells is the activity of NAD(P)H-oxidases, predominantly one encoded by the NOX4 gene. Irradiation with 0.1 Gy and treatment with ecDNAR led respectively to a 3-fold and 1.7 fold increase in NOX4 mRNA, while oxidized DNA stimulated transcription 5-15 fold compared with unoxidized DNA.
Also in previous work by the Russian team, the bystander effect involves DNA-binding to Toll-like receptor TLR9. This was confirmed by blocking the TLR9 response with chloroquine and oligonucleotide 2088, which suppressed the increase in ROS production and eliminated the effects of ecDNAR.
The team suggested that the bystander effect-like properties of ecDNAR and oxidized DNA may be used for the development of novel anti-tumour therapy that may stimulate cell death without actual irradiation, or synergistically with reduced irradiation doses.
Secondary photoelectron effects
Another way low dose ionization radiation can be amplified and appear as bystander effects is through scattering of photons through the tissues. Photons or particles can bounce off one target atom and strike another, generating a further free electron (see Box 2).
A research team at the Maria Sklodowska-Curie Memorial Cancer Centre and Institute of Oncology Gliwice Branch, Poland investigated direct and bystander effects induced by scattered radiation in two human cell lines – normal bronchial epithelial cells BEAS-2B and lung cancer epithelial cells A549 – placed in a bath of water at different depths and subjected to irradiation by 6 MeV photon beam or 22 MeV electron beam (5Gy maximum dose), and examined for apoptosis and micronucleated cells [16].
They found that for electron radiation both the numbers of apoptotic and micronucleated cells were greater than expected from the corresponding received dose, and the discrepancy between observed and expected becomes larger with increased medium depth. At a depth of 15-17 cm, the observed was ten times the expected, while micronucleated cells was about 2-3 fold. For photon radiation the biological effect did not differ significantly from expected value because photon radiation penetrates the medium better. When cells were placed outside the radiation field or under a shield, differences from expected dose were also found for both photon and electron, but no depth dependence was observed. For cells exposed outside the field of the photon beam, apoptosis was again about 7-10 fold the expected while micronuclei formation was 4-5 fold. For shielded cells under photon irradiation, apoptosis was about 3-fold while micronuclei was about 1.2-fold. For cells exposed outside the radiation field of the electron beam, again, a 10-fold difference from the expected, and for micronucleated cells, 1.5 to 4-fold in BEAS cells, and 4-7 fold in A549 cells. All the irradiated cell medium, when added to non-irradiated A549 cells gave a 2-fold increase in micronucleated cells and a 2-fold increase in apoptotic cells, regardless of the dose of irradiation or whether it was inside the beam, outside the beam or shielded.
Apart from the bystander effects mediated through the exposed cell medium, these experiments indicate that secondary photoelectron scattering may be involved in the biological effects of low-dose radiation. This has been suggested by research published in the early 1990s [17]. Monte Carlo track structure methods were used to illustrate the importance of low-energy electrons produced by low linear-energy-transfer radiations. These low-energy secondary electrons contribute substantially to the dose in all low-LET irradiations, and account for up to nearly 50 % of the total dose imparted to a medium when irradiated with electrons or photons. Up to 50 % of secondary electrons themselves can also undergo further scattering and to generate more free electrons. For most ionizing radiations, nearly 50 % of all ionizations are due to secondary electrons with starting energies less than 1 keV.
Implications for risk assessment, radiotherapy and radioprotection
Risk assessment and radiation protection have been based on extrapolation from known epidemiological data that mainly relate to high dose effects that assume a linear dose-response relationship even at very low doses [4]. This is clearly untenable in view of the bystander effects at low doses, which amplify the effective dose and harm caused.
The best available evidence suggests that bystander effects are mediated by ROS. ROS is well-known to be involved in general oxidative stress, with many downstream effects that mirror bystander effects: DNA breaks, genome instability, cell death, cancer, including cell senescence and aging [18], and cataracts [19]. It is notable that these effects are appearing as significant health impacts linked to the Chernobyl fallout [20] (Chernobyl Deaths Top a Million Based on Real Evidence, SiS 55). The pro-nuclear lobby and regulators should stop denying these impacts and governments should devote much more resources to studying them instead, to prevent repeating the humanitarian disaster in the wake of the Fukushima meltdown (see [21] Truth about Fukushima, SiS 55).
The involvement of ROS also suggests that antioxidant interventions should be considered as a mitigation of bystander effects in those exposed or still being exposed to the Fukushima and Chernobyl fallouts. This is a matter of some urgency. Among the most promising findings are the well-known benefits of green tea in cancer prevention (see [22] Green Tea Against Cancers, SiS 33), and its many antioxidants polyphenols that probably account for reducing risks of heart disease, cancers, Alzheimer’s obesity, arthritis, diabetes, and a host of other conditions associated with oxidative stress (see [23] Green Tea, The Elixir of Life? SiS 33). New research from the Radiation and Cancer Therapeutics Lab at Jawaharlal Nehru University, New Delhi, and the Central University of Gujarat in India indeed shows that one of the main green tea polyphenol, EGCG (epigallocatechin-3-gallate) is most efficient at protecting DNA against g-radiation induced breaks both inside and outside the cell, and also protects cells against radiation-induced cell death, lipid peroxidation and membrane damage (see [24] Green Tea Compound for Radioprotection, SiS 55).
As far as cancer radiotherapy is concerned, the bystander effects mean that the radiation beam will cover a wider area than the physical beam, and the potential harm may outweigh the presumed benefit. The same goes for diagnostic radiology, as it occurs at doses that might induce more harmful bystander effects than the potential benefit the procedure might deliver. It is also possible that antioxidants could offer radioprotection against these procedures.
http://www.i-sis.org.uk/Bystander_Ef...tiply_Dose.php
-
02-20-2014, 07:16 PM #196AprilGuest
Worst Spill in 6 Months at Stricken Japanese Nuclear Plant
By MARTIN FACKLERFEB. 20, 2014
TOKYO — About 100 tons of highly radioactive water leaked from one of the hundreds of storage tanks at the devastated Fukushima nuclear plant, its operator said on Thursday, calling it the worst spill at the plant in six months.
The operator, the Tokyo Electric Power Company, said the leak, discovered on Wednesday and stopped on Thursday, happened far enough from the plant’s waterfront that none of the radioactive water was likely to reach the Pacific Ocean, as has happened during some previous spills. Still, the incident was an uncomfortable reminder of the many mishaps that have plagued the containment and cleanup efforts at the plant, as well as the hundreds of tons of contaminated groundwater that still flows unchecked into the Pacific every day.
The company, known as Tepco, said it had traced the latest leak to a pair of valves that were left open by mistake.
The leaked water was among the most severely contaminated that Tepco has reported in the aftermath of the March 2011 disaster at the Fukushima Daiichi plant, when damage caused by an earthquake and a tsunami led to meltdowns in three of the plant’s reactors. Each liter of the water contained, on average, 230 million becquerels of particles giving off beta radiation, the company said. About half of the particles were likely to be strontium-90, which is readily taken up by the human body in the same way as calcium, and can cause bone cancer and leukemia.
 Launch media viewer
Launch media viewer
The Tokyo Electric Power Company said it had traced the latest leak to a pair of valves that were left open by mistake. Tokyo Electric Power Co., via Associated Press That means the water was about 3.8 million times as contaminated with strontium-90 as the maximum allowed under Japan’s safety standards for drinking water. It also showed levels much more radioactive than a worrisome groundwater reading that Tepco announced earlier this month. That reading — five million becquerels of strontium-90 per liter — which was detected at a location closer to the ocean than the latest spill, prompted criticism of Tepco because the company waited five months to report it publicly.
Critics have assailed the company since the accident, saying it has been slow to acknowledge problems at the stricken plant and has disclosed too little information about the conditions inside. Even so, the government has left the company largely in charge of the cleanup work there.
Tepco has struggled to deal with the hundreds of tons of groundwater that seeps each day into the plant’s damaged reactor buildings, where it is contaminated by the melted nuclear reactor cores. To keep the radioactive water from running into the Pacific, the company must pump it out of the reactor buildings and store it in rows of huge tanks it has erected on the plant’s grounds.
So far, Tepco said, about 340,000 tons of water has accumulated in the tanks, enough to fill more than 135 Olympic-size swimming pools. A ton of water is equivalent to about 240 gallons.
http://www.nytimes.com/2014/02/21/wo...a.html?hp&_r=0
-
02-20-2014, 07:24 PM #197AprilGuestThis should be all over the news...
Airborne plutonium detected outside troubled U.S. nuclear facility — Expert: ‘Radiation event’ appears to have occurred, leading to a release; “Levels are highest ever detected” around site
Feb. 19, 2014: Traces of radiation have been found approximately half a mile northwest of the Waste Isolation Pilot Plant [...] Tests by the Carlsbad Environmental Monitoring and Research Center [...] showed evidence of trace amounts of americium and plutonium on an air filter Wednesday afternoon [...] [CEMRC director Russell] Hardy said even though trace amounts of radiation were detected between Tuesday and Sunday, it’s important to note that radiation levels have been “very low and are well below any level of public and environmental hazard.”
Press release from Carlsbad Environmental Monitoring and Research Center, Feb. 19, 2014: “The levels detected during this time period are higher than the normal background levels of radioactivity from transuranic elements commonly found at this sampling station, thus their presence during this specific time frame appears to indicate a small release of radioactive particles from the WIPP underground exhaust shaft in the brief moments following when the radiation event occurred and when the WIPP ventilation system shifted to the filtration mode.”
AP, Feb. 19, 2014: [...] radiation [is] in the air a half-mile from the site [...] radioactive isotopes americium and plutonium [...] [Hardy] says the levels are the highest ever detected at or around the site but are far below those deemed unsafe by the Environmental Protection Agency. The readings came after a radiation alert over the weekend from an underground sensor at the site. Hardy says readings will be completed next week on filters collected from that underground sensor and an air monitor closer to the plant.
Initial ENE report from Sunday: Alarm after 'unusually high' radiation levels at U.S. nuclear site -- Gov't: "We've never seen a level like we are seeing... I can't tell you the amount" -- Could be Plutonium -- 'Unclear' how much radiation released -- Unprecedented event (VIDEO)
See also: LA Times: Expert says 'radiological process' may have forced material out of container at US nuclear site, "Could be a mess"; Officials saying little about extent of problem -- Levels remain too high to let in non-essential personnel -- Air monitors not allowed to collect filters
-
02-21-2014, 10:04 AM #198AprilGuest
TV: It’s a “record-high” leak at Fukushima, extraordinarily radioactive — Almost 8,000,000 times limit — Even more toxic since it was from early in disaster — Alarm went off 9 hours before, but disregarded — Now checking for other leaks — “News came as a shock” (VIDEO)
NHK, Feb. 20, 2014: Record-high tainted water leak at Fukushima plant [...] the leaked water contained an extraordinarily high 230-million becquerels per liter of beta-ray emitting substances, consisting mainly of strontium 90. The level is about 7.6 million times the government’s permissible standard [...] the highest level of radioactive substances detected so far in the series of tank leaks at the site. [...] Officials say they managed to stop the leak [...] 6 hours after the problem was first discovered. [...] The Nuclear Regulation Authority hasPublished: February 20th, 2014 at 9:54 pm ET
By ENENews
 instructed TEPCO to check other tanks for possible leakages.
instructed TEPCO to check other tanks for possible leakages.
Kyodo, Feb. 20, 2014: More than nine hours before the leak was recognized, an alarm indicating a rise in the tank’s water surface level was issued. But workers thought the device was out of order and also could not find leaks when they patrolled the area [...]
NHK, Feb. 20, 2014: [Tepco] found the water contained 240 million becquerels per liter of beta-ray emitting substances, including strontium. [...] an alarm went off more than 9 hours before the leak was spotted, signaling an increase in the tank’s water level. But the tank’s water-level gauge showed a sharp drop, leading workers to believe that the alarm sounded due to the malfunction of the gauge.
AP, Feb. 20, 2014: [Tepco] said the leak involved partially treated water from early in the disaster at the plant, meaning it was more toxic than previous leaks.
Al Jazeera, Feb. 20, 2014: Leaked water is almost 8 million times [legal limit for ocean release].
Tepco: “We think that the amount that leaked out of the barrier is 100 tons [...] As far as we have confirmed at this point there’s no drain nearby [...] We think there’s been no leak into ocean.”
Masakazu Yabuki, head of Fukushima Pref. fishermen’s group: “This news came as a shock.”
Watch NHK’s broadcast here
http://enenews.com/tv-record-high-le...-alarm-went-of
-
02-21-2014, 10:10 AM #199AprilGuestYeah ...right ....we can believe that.....after all the lies......"We think there’s been no leak into ocean.”
-
02-21-2014, 10:12 AM #200AprilGuest
Senior Scientist: Fukushima radiation already on West Coast of N. America — We don’t know how much is coming or how fast it’s moving, situation ‘evolving’ — Levels will continue to rise for years — Unprecedented event for Pacific, largest ever radioactive release into ocean (VIDEO)
Center for Marine and Environmental Radiation, Woods Hole Oceanographic Institution (Emphasis Added): The release of radioactive contaminants from Fukushima remains an unprecedented event for the people of Japan and the Pacific Ocean. [...] Some Fukushima radiation has already begun to appear on the West Coast of North America and is expected to peak in most places between 2014 and 2015. [...] continued leaks from the Fukushima Dai-ichi nuclear power plant [...] sparked fears of wide-ranging impacts to the marine ecosystem and human health. Despite concerns, there is no U.S. government agency monitoring the spread of low levels of radiation from Fukushima along the West Coast and around the Hawaiian Islands—even though levels are expected to rise over coming years. Whether you agree with predictions that levels of radiation along the Pacific Coast of North America will be too low to be of human health concern or to impact fisheries and marine life, we can all agree that radiation should be monitored [...]Published: January 15th, 2014 at 9:10 pm ET
By ENENews

Woods Hole Oceanographic Institution, Jan. 14, 2014 (Emphasis Added): Although [Woods Hole Oceanographic Institution marine chemist Ken] Buesseler does not expect levels to be dangerously high in the ocean or in seafood as the plume spreads 5,000 miles across the Pacific, he believes this is an evolving situation that demands careful, consistent monitoring to make sure predictions are true. “I’m particularly excitedabout finding support for sampling key locations along the West Coast multiple times throughout the coming two years, because radioactivity levels are expected to be increasing,” he says.
Crowdsourcing Fukushima, Jan. 14, 2014 (Emphasis Added): [...] It’s become the largest accidental source of radioactive isotopes to the ocean in history. […] We know there’s contaminated water coming out of there even today […] What we don’t really know is how fast and how much is being transported across the Pacific. Yes, models tell us it will be safe, yes the levels we expect off the US West Coast and Canada we expect to be low, but we need measurements — especially now, as the plume begins to arrive along the West Coast and will actually increase in concentrationover the next 1 to 2 years. Despite public concern about the levels, no public agency in the US is monitoring the activities in the Pacific. […] Without careful, extensive, consistent monitoring, we’ll have no way of knowing how much radiation from Fukushima is reaching our shores, and how it could affect life in the ocean […]
Watch the message from Buesseler here


 29Likes
29Likes LinkBack URL
LinkBack URL About LinkBacks
About LinkBacks




 Reply With Quote
Reply With Quote
 Yuliyan Velchev/Shutterstock
Yuliyan Velchev/Shutterstock 


Antifa crackdown: Trump signs EO designating far-left group as...
09-28-2025, 07:48 AM in General Discussion